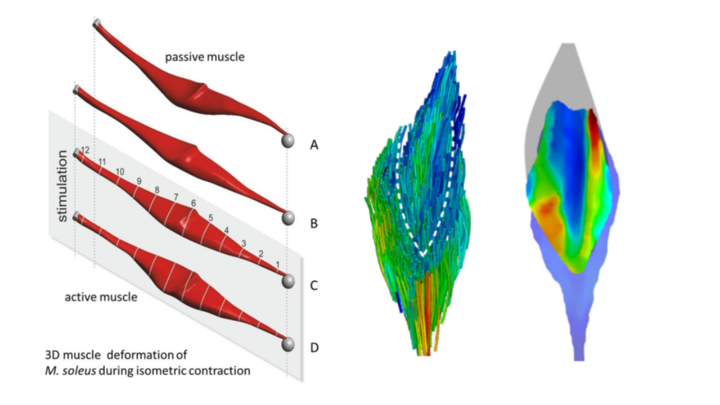
Parameter identification, simulation and verification
Skeletal muscles are usually tightly packed in muscle bundles (e.g., calf) in our body and cannot freely deform during contraction, but must interact with surrounding muscle tissue, among other things. The intermuscular transmission of force in longitudinal and transverse directions affects the force capacity and deformation of the single muscle (Siebert et al. 2016, Wick et al. 2018, Reinhardt et al. 2016). This hinders muscle coordination and differentiated force generation. Nevertheless, muscles need to perform complex movement tasks in a differentiated manner in everyday life. This project aims to gain a deeper understanding of muscle packing under the premise of force and velocity generation by studying the architecture, three-dimensional deformation, and force development of muscle packs.
Based on the preliminary work on 3D finite element (FE) modeling of the soleus muscle (SOL), the description will be extended to a muscle package (SOL, gastrocnemius muscle (GM) and plantaris muscle (PLA) of Oryctolagus caniculus (rabbit)). Three consecutive work packages are planned, in which a close integration between experiment and modeling is foreseen. In work package 1, outstanding experimental work as well as work steps for the validation of the FE model on the SOL will be completed. In work package 2, the modeling approach on the GM, which is much more complex than the SOL in its muscle architecture, fiber distribution, and contraction properties, will be validated. In work package 3, the first 3D model of a muscle package (SOL, GM, PLA) will be created and validated using the previous results.
To implement these priorities, targeted experiments will be conducted to determine the mechanical properties and structural parameters of the GM and PLA (Siebert et al. 2015, Böl et al. 2015). This includes determination of active muscle properties, passive dynamic tissue properties (Böl et al. 2012, Böl et al. 2013, Siebert et al. 2015), and 3D muscle architecture (Schenk et al. 2013, Wick et al. 2018). To characterize the interaction of adjacent muscles, longitudinal and transverse (Reinhardt et al. 2016) force transmission is experimentally assessed. Due to the different dynamics of the combined compartments, model extensions regarding fatigue and history dependence (Till et al. 2008, Till et al. 2010, Rode et al. 2008) of muscle force are necessary. For model validation, simultaneous measurement of force and 3D deformation (optical measurement system) of single muscles (Böl et al. 2013, Böl et al. 2015) as well as of the muscle bundle at different contraction forms is an essential prerequisite. Perspectively, in the fundamental area, questions about the function and design of muscles, muscle bundles and body segments will be addressed. In medical applications, models of muscle packages can be used to predict functional effects of surgery in neuromuscular diseases. Predictions of circulatory disturbances and microtrauma following eccentric loading are possible via knowledge of local pressures and stress concentrations.
Literature:
- Borsdorf, M., Papenkort, S., Böl, M., Siebert, T., Influence of muscle length on the three-dimensional architecture and aponeurosis dimensions of rabbit calf muscles, Journal of the Mechanical Behavior of Biomedical Materials (2024), doi106452 [link]
- Walter, F., Seydewitz, R., Mitterbach, P., Siebert, T., Böl, M., 2022. On a three-dimensional model for the description of the passive characteristics of skeletal muscle tissue. Biomech Model Mechanobiol. [link]
- Siebert, T., Screen, H.R.C., Rode, C., 2021. Computational modelling of muscle, tendon, and ligaments biomechanics, in: Jin, Z., Li, J., Chen, Z. (Eds.), Computational Modelling of Biomechanics and Biotribology in the Musculoskeletal System, Second Edition ed. Elsevier, Cambridge, pp. 155-186.[link]
- Rockenfeller, R., Günther, M., Stutzig, N., Haeufle, D.F.B., Siebert, T., Schmitt, S., Leichsenring, K., Böl, M., Götz, T., 2020. Exhaustion of skeletal muscle fibers within seconds: incorporating phosphate kinetics into a Hill-type model. Frontiers in Physiology, section Striated Muscle Physiology.doi:10.3389/fphys.2020.00306 [link]
- Schenk, P., Papenkort, S., Böl, M., Siebert, T., Grassme, R., Rode, C., 2020. A simple geometrical model accounting for 3D muscle architectural changes across muscle lengths. J Biomech.doi:10.1016/j.jbiomech.2020.109694 [link]
- Seydewitz, R., Siebert, T., Böl, M. (2019). On a three‑dimensional constitutive model for history effects in skeletal muscles. Biomechanics and Modeling in Mechanobiology. doi.org/10.1007/s10237-019-01167-9 [link]
- Wick, C., Böl, M., Müller, F., Blickhan, R., Siebert, T., 2018. Packing of muscles in the rabbit shank influences three-dimensional architecture of M. soleus. J Mech Behav Biomed Mater 83, 20-27. [link]
- Siebert, T., Tomalka, A., Stutzig, N., Leichsenring, K., Böl, M., 2017. Changes in three-dimensional muscle structure of rabbit gastrocnemius, flexor digitorum longus, and tibialis anterior during growth. J Mech Behav Biomed Mater 74, 507-519. [link]
- Reinhardt, L., Siebert, T., Leichsenring, K., Blickhan, R., Böl, M., 2016. Intermuscular pressure between synergistic muscles correlates with muscle force. J Exp Biol 219, 2311-2319.[Link]
- Böl, M., Leichsenring, K., Siebert, T., 2016. Effects of growth on muscle, tendon and aponeurosis tissues in rabbit shank musculature. Anat Rec 300, 1123-1136.[Link]
- Böl, M., Leichsenring, K., Ernst, M., Wick, C., Blickhan, R., Siebert, T., 2015. Novel microstructural findings in M. plantaris and their impact during active and passive loading at the macro level. J Mech Behav Biomed Mater 51, 25-39.[Link]
- Siebert, T., Leichsenring, K., Rode, C., Wick, C., Stutzig, N., Schubert, H., Blickhan, R., Böl, M., 2015. Three-Dimensional Muscle Architecture and Comprehensive Dynamic Properties of Rabbit Gastrocnemius, Plantaris and Soleus: Input for Simulation Studies. PLoS ONE 10, e0130985.]Link]
- Böl, M., Ehret, A.E., Leichsenring, K., Weichert, C., Kruse, R., 2014. On the anisotropy of skeletal muscle tissue under compression. Acta Biomat 10, 3225-3234.[Link]
- Böl, M., Leichsenring, K., Weichert, C., Sturmat, M., Schenk, P., Blickhan, R., Siebert, T., 2013. Three-dimensional surface geometries of the rabbit soleus muscle during contraction: input for biomechanical modelling and its validation. Biomech Model Mechanobiol 12, 1205-1220.[Link]
- Schenk, P., Siebert, T., Hiepe, P., Gullmar, D., Reichenbach, J.R., Wick, C., Blickhan, R., Böl, M., 2013. Determination of three-dimensional muscle architectures: validation of the DTI-based fiber tractography method by manual digitization. J Anat 223, 61-68.[Link]
- Böl, M., Kruse, R., Ehret, A.E., Leichsenring, K., Siebert, T., 2012. Compressive properties of passive skeletal muscle - The impact of precise sample geometry on parameter identification in inverse finite element analysis. J Biomech 45, 2673-2679. [Link]